Faller Packaging optimises secondary packaging procurement for leading pharmaceutical manufacturers
More orders in ever-smaller batch sizes and with short lead times: this increases the degree of complexity that already exists in the pharmaceutical industry’s entire supply chain. It also led to severe production bottlenecks for one of the world’s leading manufacturers. Even the procurement of the required secondary packaging was unnecessarily costly and time-consuming. The packaging specialist company Faller Packaging helped the manufacturer make this process faster and more reliable, cost-effective and sustainable.
The pharmaceutical and healthcare industry is one of the largest and most dynamic growth markets in the world. There are various reasons for this, such as a rapidly growing world population and the demographic shift toward ever-older societies, factors that also increase the demand for medicines and other pharmaceutical products. For most pharmaceutical manufacturers, however, the market environment has also become much more volatile due to increasing international competition, new forms of therapy and dosage and the trend towards greater individualisation. Time-to-market is also decreasing, resulting in more and more products coming onto the market at ever-shorter intervals.
This has serious implications for the industry’s production concepts: batch sizes of individual orders are becoming much smaller, but order quantities are increasing, resulting in more frequent changes and changing demands in production processes. Shorter lead times and a high level of cost efficiency are also prerequisites for market success. All this means that the entire supply chain for the manufacture of pharmaceutical products is becoming much more complex – also in terms of the procurement of the relevant packaging.
Increasing article variety, decreasing predictability
The increasing individualisation of pharmaceuticals is leading to a fast-growing variety of articles. Secondary packaging products such as folding cartons, labels and leaflets are particularly affected since they are often specially designed for different customers and countries. It is becoming more difficult for pharmaceutical companies to correctly estimate their packaging needs in advance and to clock times and manufacturing and logistics processes accordingly. All these factors result in unnecessary, avoidable costs for storage space, transport, machine downtime, and the destruction of redundant materials.
This challenge was also addressed by a leading global pharmaceutical company that manufactures wound care products and other items at one of its plants in southern Germany. The increasing demand for its products caused the manufacturer’s site production volume to grow steadily year after year. However, the on-site capacities could not be expanded to match the demand. More and more orders with small batch sizes and short lead times led to bottlenecks in production, caused unnecessary costs and severely restricted the manufacturer’s ability to deliver reliably.
Complex ordering and checking processes simplified
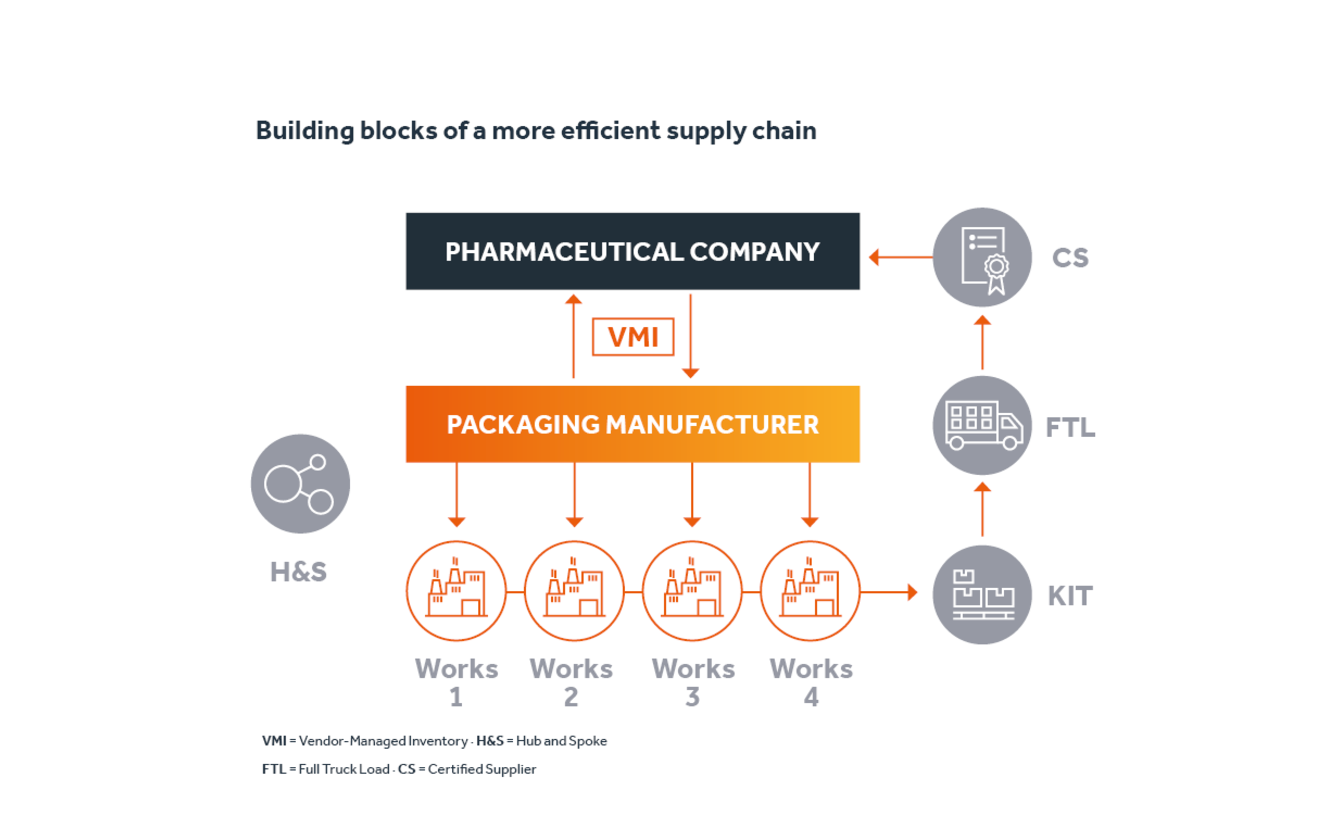
In the past, the pharmaceutical company had always relied on Faller Packaging to procure its secondary packaging materials. The packaging specialist, headquartered in Waldkirch in Baden-Wuerttemberg, supplied folding cartons, labels and leaflets from a single source. However, the customer always submitted a separate order for each product – a labour-intensive and unnecessarily complicated process.
The pharmaceutical manufacturer also had to subject all incoming goods to a strict quality inspection – including the packaging components. The area where the supplies to be inspected were temporarily stored was limited and regularly overfilled, however, resulting in allocation errors and expensive delays caused by the excessive workload of the incoming goods inspection department.
Together with Faller Packaging, the customer initiated procedures to make the procurement process faster and more straightforward and efficient. In the first step, the orders for folding cartons, labels and package leaflets were bundled by product – so instead of three orders, Faller Packaging only received one. The packaging specialist could then optimally distribute the KIT orders to its internal production capacities in the various plants, produce the items and subsequently put them together on pallets, even before they were shipped to the customer.
Complete control over the inventory
The pharmaceutical manufacturer also introduced a vendor-managed inventory (VMI) together with its supplier. As a result, Faller Packaging assumed complete responsibility for the inventory of its products at the customers, sharing the inventory and sales figures of the respective items to this end. Instead of receiving orders for individual products, Faller Packaging now obtains information from this data regarding how many items of which product are needed and when, and then initiates the corresponding production. The specialist company can now deliver the relevant orders to the customer at short notice and significantly relieve the on-site storage capacities. The ability to plan for raw materials and capacities early and deploy them efficiently also saves time and costs. The pharmaceutical manufacturer is also able to outsource a significant part of the complexity involved in procuring secondary packaging materials, enabling it to focus more on its actual value creation.
As a certified supplier, Faller Packaging took over the inspection of the delivered products, which also optimised the quality assurance process. This meant that it was no longer necessary for the pharmaceutical manufacturer to conduct repeated in-house inspections and testing. After a brief incoming goods inspection, the packaging required for production was delivered to the company’s packaging line “right on time”, and this alone generated massive time and cost savings. The results for the customer? The support provided by Faller Packaging reduced lead times in the pharmaceutical company’s production by an average of 65% and cut its expenses by around €400,000.
The detailed case study can be found on our website