Faller Packaging offers sustainable, production-ready packaging for pens, syringes and kits
New active ingredients for weight reduction mark a turning point in obesity therapy. They can be applied conveniently with auto-injectors or pens, even in weekly doses. This makes the procedure easier for patients, encouraging them to inject the medication themselves at home. Manufacturers therefore need to rethink not only the content, but also the packaging, because resistance to breakage, intuitive handling, space-saving formats, and sustainable materials are becoming increasingly important.
Know-how for special requirements
Faller Packaging specialises in pharmaceutical and healthcare packaging and also offers modular systems for pens, syringes and complex kits. “Standard packaging often reaches its limits, especially for devices such as weight-loss syringes,” says Faller Product Manager Benjamin Rist. “Our designs combine format, protection requirements and user needs.”
However, the requirements are high, since solutions are needed that offer more than just protection for pens and syringes and for sets with vials, ampoules and accessories. The packaging should be simple to incorporate into existing production lines without compromising user convenience. “This can only be achieved with a tailor-made design that precisely matches the product, process, and regulatory requirements,” explains the Product Manager. “Our developers have the necessary know-how and pharmaceutical expertise.”
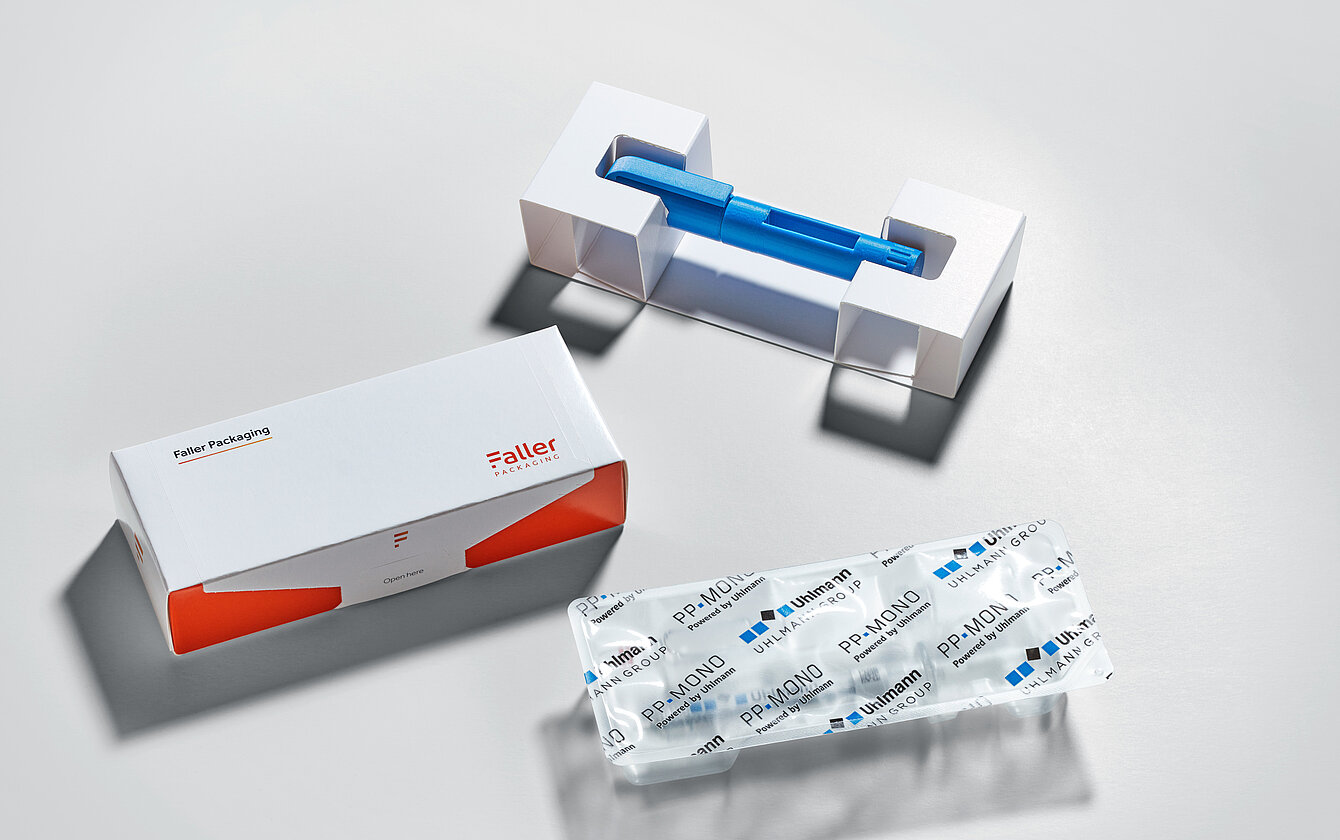
Faller Packaging cooperates with Uhlmann Pac-Systeme – a specialist in packaging technologies for parenterals to provide a comprehensive solution to the complex challenges. This cooperation combines material expertise and process and industry knowledge. Together, the partners develop solutions that can be seamlessly integrated into existing packaging lines and are precisely tailored to the specific conditions and processes in pharmaceutical production. Uhlmann Pac-Systeme’s portfolio includes the PTC 200 packaging line, which was specially developed for parenteral products. It enables the flexible processing of paper and plastic trays and their packaging in folding cartons – while at the same time ensuring the safe and gentle handling of sensitive medical devices such as pens, vials, ampoules and syringes.
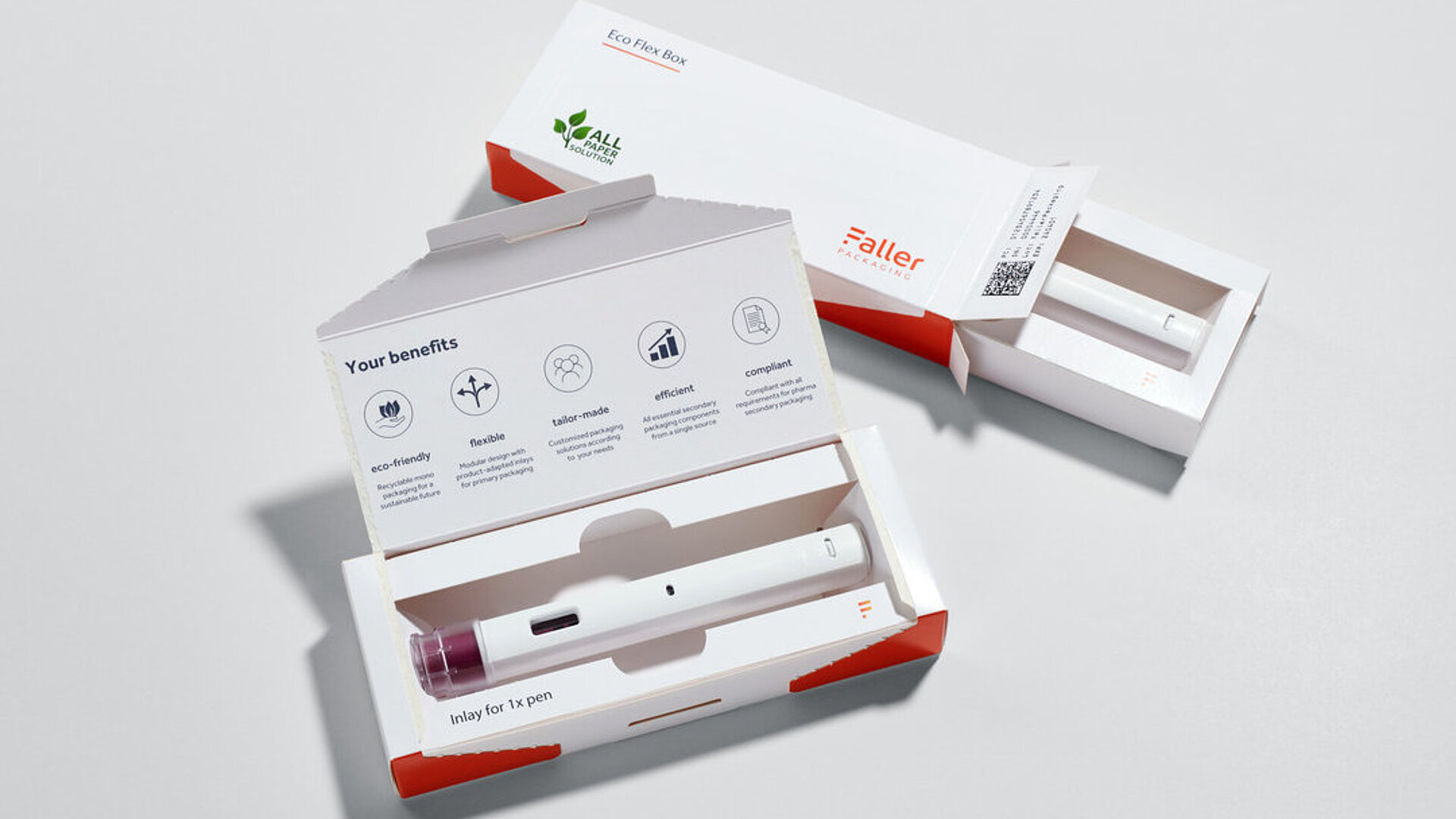
Sustainability starts with the inlay
Faller Packaging covers different application scenarios with two packaging technologies: SLTO (Side-Loading Top-Opening) and TLTO (Top-Loading Top-Opening). However, the customisable inlays are a central component of the packaging. They’re either made from plastic-free, fibre-based monomaterial or from fibre moulding, a stable cellulose alternative to plastic. Depending on requirements, both variants can be used to produce recyclable individual packaging or multipacks. “For a current customer project, for example, we’re replacing an inlay that was previously plastic-based with a cardboard-based alternative,” explains Benjamin Rist.
Get off to a faster start with pre-assembled packaging
Faller Packaging goes one step further, however, supporting pharmaceutical manufacturers even before the start of series production of a new drug: With the PrePackaging Service, customers can test packaging early on in the development process, in clinical trials, for example, or market launches. Flexible packaging concepts are particularly important at this stage, as investment risks and fluctuating demand make planning more challenging. The concept involves ready-to-use, prefabricated packaging systems that can be manually assembled in small quantities and later processed automatically without any design changes.
Faller Packaging’s development department supports projects from phase 3 of the clinical trials. The advantages here for pharmaceutical companies include shorter time-to-market, calculable costs, reduced development effort and reliable pharmaceutical compliance. This service is another example of how Faller Packaging meets complex secondary packaging requirements in a practical and tailored way, using its unique technical expertise.